Seq&Treat

What is this project?
 brings next-generation TB care to underserved communities. The Seq&Treat project is designed to generate evidence and boost in-country capacity to support the global adoption of commercial, targeted next-generation sequencing (NGS) for affordable, scalable and rapid TB drug-susceptibility testing (DST).
brings next-generation TB care to underserved communities. The Seq&Treat project is designed to generate evidence and boost in-country capacity to support the global adoption of commercial, targeted next-generation sequencing (NGS) for affordable, scalable and rapid TB drug-susceptibility testing (DST).
Why are we working on it?
Drug-resistant (DR-)TB is a public health crisis – in 2018, it was estimated that 500,000 people developed TB that was resistant to rifampicin, the most effective first-line drug, and 78% of those people had multidrug-resistant TB. Yet, only 187,000 DR-TB cases were detected and reported.
The spread of DR-TB is exacerbated by the lack of rapid, accurate diagnostic tests for comprehensive DST. Culture-based DST is the current standard of care, but it is slow and comes with significant biosafety hazards and minimal potential for cost reductions. Targeted NGS has the potential to revolutionize the DST landscape as it can provide faster, safer and more comprehensive results that can inform clinical decision-making for existing, repurposed and new DR-TB treatment regimens.
What does it involve?
Seq&Treat aims to generate clinical evidence to support WHO global guidance on the use of targeted NGS for DR-TB diagnosis so that patients can be linked to the most appropriate care as fast as possible. In addition, it establishes a WHO global clinical knowledgebase, evaluates proof-of-principle delivery models for integrating targeted NGS into existing diagnostic work streams, and facilitates inclusion of recommended NGS solutions into global procurement mechanisms and adoption by low- and middle-income countries.
Overview of technology selection and evaluation
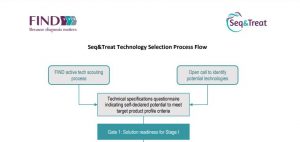
The technology selection process for Seq&Treat entailed a combination of active technology scouting efforts and an open call for expressions of interest on the FIND website prior to start of the project*. The technology evaluation process for Seq&Treat involves a rigorous stage-gated approach, comprising three gates within Output 1 and Output 3 associated with milestones, to determine progress to subsequent activities. The stage-gating process is supported by a Technical Review Committee (TRC) composed of independent experts.
| Seq&Treat technology selection process flow | Terms of reference for Technical Review Committee |
 |
 |
___________
![]() *More information for new manufacturers developing end-to-end NGS solutions for TB
*More information for new manufacturers developing end-to-end NGS solutions for TB
What do we expect to achieve?
Bringing the power of sequencing closer to communities with a high burden of DR-TB will enable rapid diagnosis and comprehensive treatment guidance in the short term, as well as catalyze an eventual decrease in the transmission of DR-TB, and reductions in DR-TB incidence and prevalence.
What is the timescale?
Seq&Treat will run for 4 years, from 2019 to 2022.
Partners and funding
Seq&Treat is a FIND project, funded by Unitaid, which builds on earlier sequencing work that has been supported by the Bill & Melinda Gates Foundation, the Australian government, and UK aid from the British people.
More information
For more information, please email info@finddx.org.
Quick links

