Sequencing
GOAL: Enable genomics for improved surveillance and diagnosis
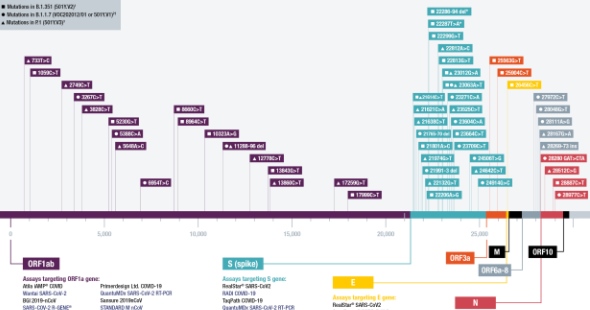
Sequencing is a process used to understand the genetic makeup of a biological organism; next-generation sequencing (NGS) collectively describes various high-throughput, rapid, and scalable sequencing technologies that are used to determine the DNA or RNA sequence of the complete genome or part of a genome. NGS enables rapid identification of unknown pathogens, discovery of novel genetic variations, and improved understanding of disease-causing pathogens, to inform the development and utility of tests, treatments, and vaccines. An increasingly critical application of sequencing is genomic surveillance, which uses sequencing data to identify outbreaks of existing or novel pathogens, and to understand how pathogens are introduced and spread through a population. The goal of our sequencing programme is to establish the use of NGS technologies for the diagnosis of drug-resistant TB, and genomic surveillance of COVID-19, TB, AMR, and other outbreak-prone diseases. Our anchor project is designed to generate evidence and boost in-country capacity to support the global adoption of targeted NGS for affordable, scalable, and rapid TB drug-susceptibility testing. Bringing the power of sequencing closer to communities with a high burden of drug-resistant TB will enable rapid diagnosis and comprehensive treatment guidance and catalyse an eventual decrease in its transmission. As part of the ACT-Accelerator Diagnostics Pillar Genomic Surveillance Working Group, we are strengthening genomic surveillance capacity in low- and middle-income countries (LMICs) as part of the COVID-19 response. We are also working on sequencing for AMR surveillance and viral haemorrhagic fevers.
Our focus is on enabling end-to-end sequencing solutions – from sample processing to bioinformatics and interpretation – for disease surveillance and diagnostics in LMICs.
Workstreams:
- Generate clinical evidence to support WHO global guidance and policy on the use of targeted NGS for drug-resistant TB diagnosis, so that patients can be linked to the most appropriate care as fast as possible
- Enable tools for analysis and interpretation of sequencing data to inform action, including variant identification and prediction of drug resistance
- Provide technical and operational guidance for country capacity building and implementation of sequencing-based surveillance and diagnosis
- Inform industry and donors on technology and access gaps via landscapes, target product profiles, capacity mapping and needs assessments
Indicative deliverables:
- First WHO-endorsed global TB mutations catalogue to predict drug resistance from genetic data generated by molecular and sequencing-based tests
- Implementation guide and roadmap for country planning and adoption of sequencing for infectious disease surveillance in LMICs, with an initial focus on drug-resistant TB
- Global capacity mapping framework to support implementation of genomic surveillance, resource allocation, and country prioritization for COVID-19 and beyond
- Operational roadmap to inform investment case to strengthen sequencing and bioinformatics capacity in LMICs for surveillance, as part of the ACT-Accelerator Diagnostic Pillar Genomic
Surveillance Working Group
GENOMICS & SEQUENCING TECHNICAL REPORTS
| Read our report Sequencing for antimicrobial resistance surveillance |  |
Page last updated 13 Apr 2022
Quick links





