Request for proposals

This RFP is now closed
Accelerating the availability of affordable SARS-CoV-2 self-tests
- The Foundation for Innovative New Diagnostics (FIND) is leading a Request for Proposals (RFP) to accelerate the availability of low-cost self-testing solutions for SARS-CoV-2 in low- and middle-income countries (LMICs).
- This RFP has been prepared in the context of the Access to COVID-19 Tools Accelerator (ACT-A) Diagnostics Pillar.1 Launched by FIND, it supports the plans of two working groups within the pillar: “R&D of tests & digital tools” and “Market readiness”.
- Innovators, developers and manufacturers of in vitro diagnostics, and LMIC-based diagnostic stakeholders, are invited to submit proposals.
- Funding mechanisms are determined according to the needs of each applicant.
Background
Much progress has been made since the early days of the COVID-19 pandemic, with testing capacity in many countries having been scaled up at an unprecedented speed. Yet, in many settings, testing still remains highly centralized and is sometimes insufficient to meet demand. While countries in all regions have experienced these challenges, the needs are more acute in LMICs. Under-resourced health systems and reliance on global supply chains have often left LMICs unable to access affordable COVID-19 testing or to deliver it to those populations most in need. To address the need for decentralized COVID-19 testing in LMICs, in 2020, ACT-A launched an Expression of Interest (EOI) for late-stage product development and manufacturing scale up of low-cost professional use antigen rapid diagnostic tests (RDTs), which resulted in several awards made to manufacturers on behalf of the ACT-A Diagnostics pillar.2 In addition to the EOI, several ACT-A partners have engaged in additional activities to support the introduction and use of COVID-19 RDTs both within and outside of healthcare facilities.
In the current RFP, we seek to expand the portfolio of testing tools to further enhance countries’ pandemic response. As part of a comprehensive testing programme for SARS-CoV-2, self-administered tests could have a potential to significantly contribute to LMICs’ response to the COVID pandemic by expanding access to testing services. There is emerging thinking that self-testing options could potentially support the COVID-19 response by reducing community spread, when linked to appropriate responses for isolation, contract tracing and care.3 In addition, modelling shows that if self-testing is performed frequently, and if patients are compliant with countermeasures, it could have a greater impact on transmission than one-time testing using more sensitive but slower laboratory-based diagnostics.4 Such serial testing strategies may detect individuals with high viral loads, who may be more infectious compared to others, and this approach may also facilitate early isolation of the infectious individual, ultimately reducing transmission.
Although the United States Food and Drug Administration (FDA) has approved five tests for at-home use, their price (>$24 USD) prohibits widespread adoption in LMICs. However, low-cost technologies, initially developed for administration by healthcare professionals, could be adapted for affordable self-testing.
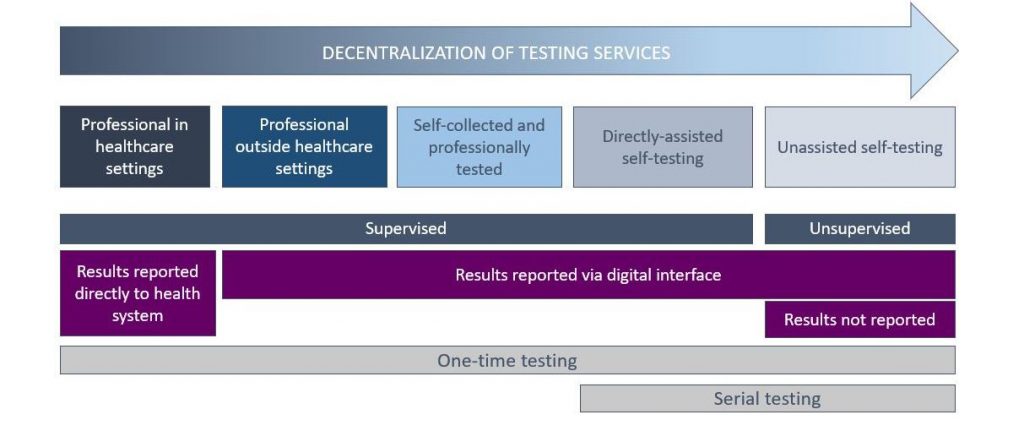
Figure 1: Situating self-testing within RDT testing approaches
_____________________________________________
1 ACT-A is a global collaboration to accelerate the development, production, and equitable access to COVID-19 tests, treatments, and vaccines. It was set up in response to a call from G20 leaders and launched by the World Health Organization (WHO), European Commission, government of France and the Bill & Melinda Gates Foundation in April 2020. The Diagnostics Pillar of ACT-A is co-chaired by FIND and The Global Fund to Fight Aids, Tuberculosis, and Malaria.
ACT-A is not a decision-making body or a new organization, but works to speed up collaborative efforts among existing organizations to end the pandemic. It is a framework for collaboration that has been designed to bring key players around the table with the goal of ending the pandemic as quickly as possible by reducing COVID-19 mortality and severe disease through the accelerated development, equitable allocation, and scaled up delivery of tests, treatments and vaccines, thereby protecting health systems and restoring societies and economies in the near term. It draws on the experience of leading global health organizations that are tackling the world’s toughest health challenges, and who, by working together, are unlocking new and more ambitious results against COVID-19. Its members share a commitment to ensure all people have access to all the tools needed to defeat COVID-19 and to work with unprecedented levels of partnership to achieve it.
ACT-A has four pillars of work: diagnostics (ACT-A Dx), therapeutics, vaccines and the health system connector. Cross-cutting all of these is the workstream on access and allocation.
“R&D of tests & digital tools” working group is led by the Bill & Melinda Gates Foundation and the Praesens Foundation; “Market readiness” working group is led by Unitaid and FIND.
2 https://archive.finddx.org/newsroom/pr-22jan21/
3 Paltiel et al., 2021: https://www.medrxiv.org/content/10.1101/2021.02.06.21251270v1
Hoehl et al., 2021: https://www.medrxiv.org/content/10.1101/2020.12.04.20243410v1
4 Chin et al., 2021: https://www.medrxiv.org/content/10.1101/2020.04.30.20087015v4.full.pdf
Larremore et al., 2020: https://pubmed.ncbi.nlm.nih.gov/33219112/
Objective
The main objective of this RFP is to accelerate the availability of accurate, affordable, quality-assured and easy-to-use SARS-CoV-2 self-testing solutions suitable for LMICs markets. In vitro diagnostics (IVD) developers and manufacturers with late-stage self-testing solutions or those with proven professional use technologies who are working on self-testing solutions are encouraged to apply.
In light of the higher risk of user error with self-tests, compared with professional use tests, a central focus of this RFP is to address the availability of easy to use self-testing solutions, achieved through: (1) product design (e.g., easy to obtain samples, intuitive assay performance and interpretation, accessible instructions) and (2) supporting tools. The specific activities supported by FIND as part of this initiative will vary according to the needs of each applicant. Please, refer to the Scope section below for examples of specific activities supported under this RFP.
Importantly, effectiveness of SARS-CoV-2 self-tests regimens is dependent on the tester competency and their subsequent compliance with the appropriate public health measures. Competency and compliance may be enhanced by digital tools that guide the user on how to adequately perform the test and capture and/or report data for the user, healthcare provider and surveillance systems. ACT-A Dx partners are in the process of developing product requirements for digital support tools. Based on these, FIND will facilitate partnerships between RFP respondents and digital solution developers (as needed) for comprehensive self-test solutions. Therefore, having a pre-developed digital solution is not a prerequisite for applying to this RFP. Organizations that have already developed digital tools for their self-testing product as well as those who have not are both encouraged to apply. If the manufacturer already has a digital solution, it will need to include digital connectivity through smartphone application, ability to report to country health information management systems using an onboard unique identifier or other personal data protection safeguard, linking the test to the user (e.g., QR codes, 2-D barcodes etc). Additionally, the digital solution should be open-access and comply with data ownership and governance principles. Pre-developed digital solution will be evaluated as a part of the proposal and depending of the results of the evaluation, the applicant may be asked to partner with an alternative digital solution developer identified by FIND.
Reflecting on the urgency of the COVID-19 response, we seek self-testing solutions that are in late development stage and will have a final product ready to enter prospective clinical studies by no later than Q3 2021. Independent clinical studies, funded through the RFP, will evaluate the performance of self-tests in the hands of untrained users and generate data to support regulatory submissions.
It is important to note that despite its support to the ACT Accelerator collaboration, the World Health Organization current recommendations on use of point of care testing with RDTs detecting SARS-CoV-2 antigen apply to trained operators in health care settings. WHO is interested is reviewing studies on the performance of self-test regimens in different use scenarios (screening vs SARS-CoV-2 infected suspects), the operational effectiveness of testing in various settings, and the behavioral impact of personally-managed testing results, to inform policy updates. Therefore, this RFP should not be interpreted as an endorsement of self-testing by the WHO. Complimentary activities are being funded in parallel by ACT-A to generate evidence needed to inform WHO policy (see “Complimentary activities not in scope of this RFP” section below).
Scope
We seek proposals to accelerate the development, manufacturing, and market availability of accurate, affordable, quality-assured and easy-to-use SARS-CoV-2 self-tests for use in LMICs.
This RFP aims to fill current diagnostic gaps in LMICs, namely an affordable, high-quality self-test for detecting SARS-CoV-2 infection in asymptomatic and symptomatic individuals and those with mild symptoms, in an exceptionally user-friendly format. Prototype self-testing solutions must meet the criteria listed in the high level guidance on performance and usability provided in Appendix 1. It is important to note that the applicant should provide the available evidence of test performance (e.g., performance evaluations of either the professional-use test or the self-testing solution and/or the correlation of test performance in the hands of healthcare professional vs untrained user). It is understood that the performance of the assay may depend on the population in which it was evaluated and it is likely that sensitivity in asymptomatic population may be lower than in population with symptoms.
The specific activities supported by FIND as part of this initiative will vary according to the needs of each applicant. Importantly, any support provided through this RFP will require a commitment to supplying SARS-CoV-2 self-testing solutions to LMICs under global access terms set forth by key collaborators within ACT-A .
Specific areas of support through this RFP include:
- Development of the self-testing solution. This includes, but is not limited to: optimization of the self-testing product and instructions for use, development of support packages (including printed and digital tools), tailoring of the bundled solution to target countries and settings.
- Expanding/optimizing manufacturing and distribution capacity. In addition to promising self-testing products, affordability and the ability to supply LMIC markets are paramount. Despite the nascence of the self-testing market, recommendations for serial testing could result in substantial demand. Adequate manufacturing and distribution capacity to serve LMIC demand is therefore required, and manufacturers should be willing to work with FIND to optimize production and distribution strategy to ensure affordability.
- Conduct clinical evaluations required for regulatory clearance. Alongside financial support, FIND, on behalf of ACT-A Dx, envision the design and conduct of field evaluation studies to support regulatory submissions and in-country product registration.
- Development of efficient go-to-market plan including distribution and regulatory strategies.
Investments that rapidly yield affordable self-testing solutions are particularly valuable; for example, a manufacturer of a proven professional test committed to adapting it for self-testing in LMICs. Developers of novel self-testing solutions at advanced stages of development (e.g., prototypes) are also encouraged to apply. In all cases, the expectation is that clinical evaluations should being within 4 months of executing a contract, which means that optimization of the self-testing package and usability assessments of prototype designs should have begun or will commence soon after contracting.
COMPLEMENTARY ACTIVITIES NOT IN SCOPE OF THIS RFP
Separate from this RFP, ongoing activities to support market shaping and introduction of professional use rapid tests conducted by FIND and other partners within the ACT-A diagnostic pillar (ACT-Dx) will be expanded to include self-tests, and will incorporate key studies to inform national and international policy development. Although LMICs have rapidly adopted molecular SARS-CoV-2 tests, testing using RDTs is taking longer to gain a foothold, reflecting the need to understand bottlenecks and delineate optimal strategies for decentralized SARS-CoV-2 testing. While these efforts take time, they should lay the foundations for self-administered testing in some markets. There are several potential applications or use cases for SARS-CoV-2 self-test, including serial testing in semi-closed communities (e.g., workplaces, schools, army units, refugee camps) and testing at the point of entry (e.g., border crossing, social gatherings), as well as pharmacy-based and home-based testing. The most effective use cases and algorithms have yet to be identified, but are likely to vary by country and market. To this end, FIND has begun extensive market research looking at priority use cases, the local regulatory and policy landscape, and market opportunities for self-testing. This research will be available in the coming months to inform self-testing commercialization strategies in LMICs. Additionally, FIND and Unitaid, through funding committed to ACT-A, are implementing an extensive programme to support interested countries in the development of self-testing policies and introduction of tests in priority segments identified through the needs assessment.
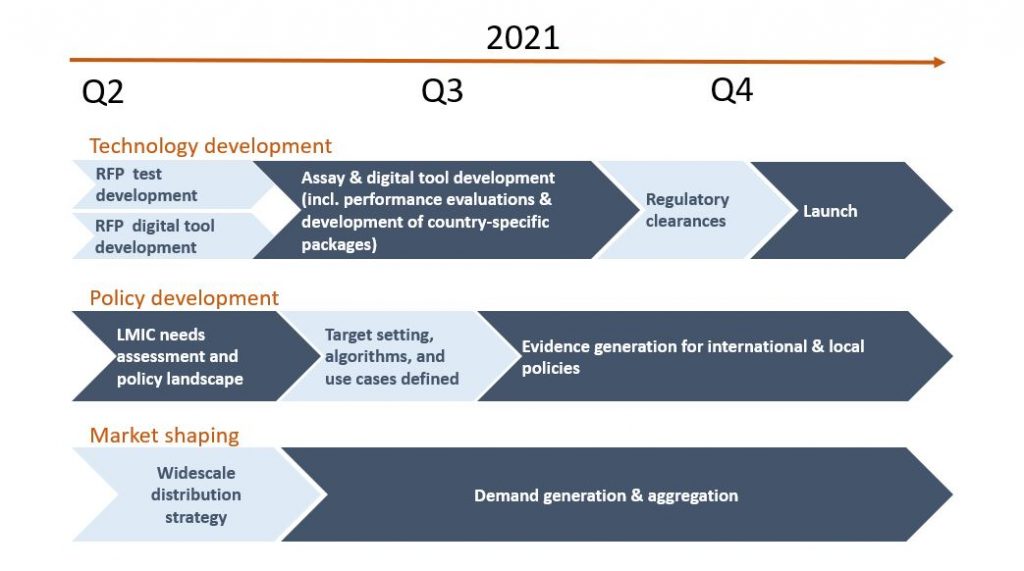
Figure 2: Overview of efforts along the value chain to catalyse and build the market for SARS-CoV-2 self-testing in LMICs
Benefits
A budget envelope of $15 million USD will be available to support 2–3 proposals that offer the best value for money. Funding negotiations will be conducted independently for each proposal and will be tailored to the applicant’s needs and the specifics of each business case. The structure of financial support may vary. Please refer to the SCOPE section above for specific areas that will be supported under this RFP.
Further, as part of the overall ACT-A initiative, this programme can facilitate connections and networking opportunities with a broad ecosystem of partners, working towards support for product introduction, commercialization, and scale-up.
Interested parties
Proposals are sought from IVD tests developers and manufacturers committed to ensuring that the benefits of newly developed tools reach not only the richest economies, but also LMICs. Specifically, companies that have a prototype self-testing solution or are in the process of amending their low-cost professional use test for self-testing are encouraged to apply. If the prototype self-testing package has a supporting digital tool developed, it should be included and described in the proposal, however, having such tool is not a requirement to apply to this RFP.
Selection criteria & proposal format
| Primary selection criteria: product affordability, reliability, ease of use, and company ability to serve LMIC demand quickly.
Proposals must demonstrate the ability to enter clinical trials with fit-for-purpose, quality-assured SARS-CoV-2 self-tests within 4 months of contract execution, with a commitment to market introduction across several LMICs by 2022 and further scale-up beyond the first year. |
In addition:
- Proposals must include a clear and meaningful value proposition for improving the supply to LMICs of SARS-CoV-2 self-testing solutions.
- Products must meet the product requirements provided in Appendix 1 and developed based on the WHO target product profiles (TPP)5 for point-of-care tests for suspected COVID-19 cases and their close contacts.
- The proposal shall include evidence supporting the test’s performance, at least for the professional-use test using a specimen type compatible with self-testing (see Appendix 1) and, if available, a correlation of test performance in the hands of healthcare professionals vs untrained user or a full evaluation of the self-testing solution.
- Although technology platforms initially designed for professional use are welcome, proposals for this RFP must reflect the unique product characteristics and custom commercialization strategies necessary for building a sustainable self-testing business in LMICs.
- If relevant, applicants should describe their digital solution or their expectations for working with a digital solution developer.
- Consideration will be given to the strengths of the commitments made to maximize equitable access, including the share of volumes committed to LMICs and proposed pricing arrangements (for more information, please refer to the FIND global access policy).
- Proposals must demonstrate experience and capacity to deliver on stated objectives.
Full details on governance, eligibility, and partner expectations are listed in Appendix 2.
_____________________________________________
Proposals should be submitted in PowerPoint, with no more than 25 slides that include the following information:
Self-test value proposition
- Value proposition and expected impact:
- An overview of the self-testing solution, including product characteristics and specifications, with a clear side-by-side reference to its alignment with the product requirements described in Appendix 1. The proposal must include the table summarizing the alignment to critical attributes (highlighted in grey in Appendix 1). Please provide explicit information about the cost per test indicating the shipping terms applied (e.g. ex works, landed costs, retail price) and currency.
- If relevant, please include as an annex an overview of the existing digital solution and how it meets the use cases specified in the Supporting Tools section of Appendix 1. Please note if the digital solution is required to read and/or report the results or intended to improve the tests performance.
- Summary of evidence supporting any claims (e.g. product performance, manufacturing, and distribution capacity)
- Timelines, including detailed stage of development, proposed activities, and deliverables
- Estimated funding need and other support requirements
- Vision for introduction and distribution of self-tests in LMICs
Company experience and track record
- High-level company background (e.g. key products and markets, distribution footprint, vision for self-testing and LMIC markets after pandemic).
- Production overview including manufacturing capacity and utilization, available capacity for self-test, expansion plans, and key inputs supply security (e.g. antibodies, swabs, etc.)
- Company / key staff detail on experience relevant to this RFP (e.g. experience with LMIC markets, self-testing and over the counter markets, and WHO PQ / EUL or other stringent regulatory authorities).
- Partnerships (if relevant) and role of each entity
- Freedom to operate and existing licensing agreements, and declaration of any relevant interests
How to apply
Submit proposals via the FIND technology scouting submission web form. Please select ‘ACT-A Dx: SARS-CoV-2 Self-test’ as the ‘Disease Area’ on the form.
Frequently asked questions
Selection process and timelines
This RFP opened on 31 March 2021; the deadline for receipt of submissions is 20 April 2021, 23:59 CET.
A commitment to a compressed timescale is required, and we anticipate funding awards and contract execution in 3 months. Following submission, a long-list of candidates will be invited to present their pitch to the review panel in a live videoconference. A short-list of candidates will then be invited to develop full proposals to be submitted for a further round of review prior to final selection. This review may include the participation of ACT-A Dx working group members who will jointly analyse RFP responses, and make decisions around the most optimal programme design and funding levels for successful applicants.
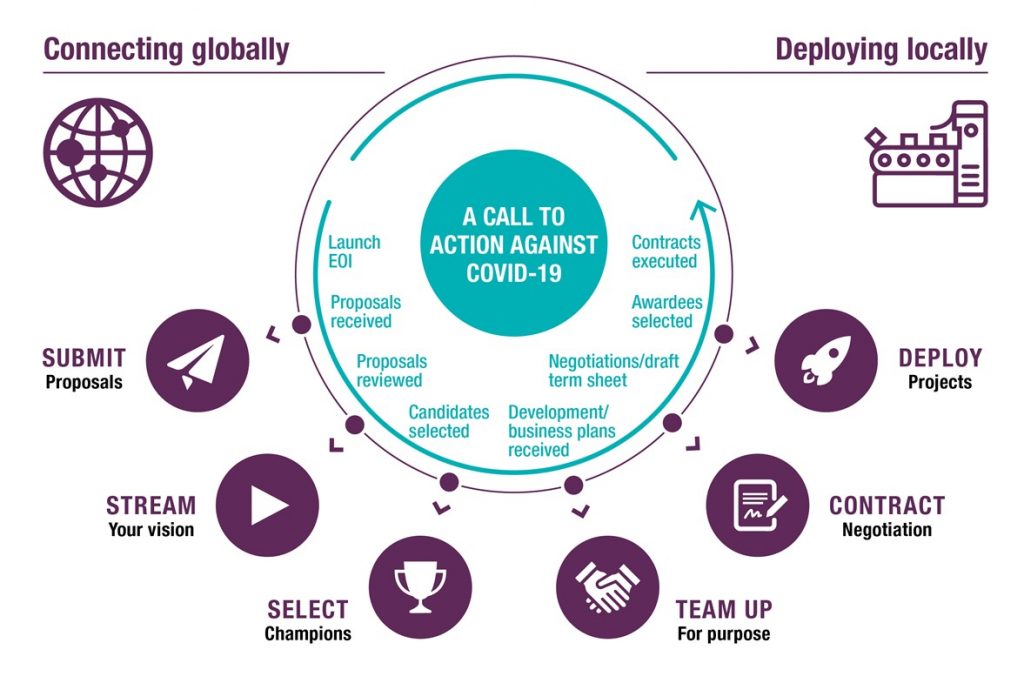
Figure 3. Selection process. Note the review may include ACT-A Dx working group members, joint analysis of RFP responses and identification and design of high priority interventions
FIND is leading this RFP through funding committed to ACT-A; management, evaluation, and selection of proposals will be conducted according to FIND governance, policies, and procedures (see Appendix 2). Proposals selected for funding negotiations will then engage with FIND, who will follow its own procedures for due diligence, contracting, and monitoring.
QUESTIONS & FURTHER INFORMATION
Please contact: rfp_acta@finddx.org
Questions will be accepted and responded to expediently while the RFP remains open.
_____________________________________________
Appendix 1: Key product requirements
Fully-developed products ready for commercialization are expected to meet the Key Product Requirements. Attributes that are considered as critical are highlighted in grey. It is understood that evidence to support claims on some of the attributes listed below (e.g. test kit stability, clinical performance) might be limited or not available at the moment of proposal submission. In that case, a potential to meet the targets will be assessed.
The product requirements listed below have been developed based on the published WHO TPPs for point-of-care tests for suspected COVID-19 cases and their close contacts, for diagnosis of acute SARS-CoV-2 infection in areas where reference assay testing is unavailable, or turnaround times obviate clinical utility. In the event that the WHO publishes a TPP that is specific for self-testing, the WHO TPP will take precedence over the requirements described below, and any product development funded under this RFP will be expected to align to it.
| Attribute | Minimal requirement | |
| Intended use | Self-administered rapid test for SARS-CoV-2 infectiousness for individuals with no symptoms and those with mild symptoms.
Several use scenarios can be considered: 1. Serial testing of individuals in congregated settings (e.g. workplaces, schools, refugee camps) in areas with ongoing SARS-CoV-2 transmission 2. Serial testing of individuals after exposure to a confirmed SARS-CoV-2 case 3. Testing at the point of entry (border crossing, social gatherings) Use cases will be refined after a detailed needs assessment is conducted in a selected subset of LMICs. |
|
| Target population | Individuals seeking to learn their SARS-CoV-2 infection status by testing themselves in suitable venues (including settings outside healthcare facilities) | |
| Lowest setting for implementation | Assisted testing in community settings (e.g., institutional settings, workplaces) | |
| Target analyte | SARS-CoV-2 biomarker of acute infection (e.g., antigen, RNA) | |
| Supporting tools | Digital and printed tools that will instruct users on each step of the testing process; developed communication system to report test results and provide post-testing counselling (smartphone application, online platform, call line) | Printed tools that will instruct users on each step of the testing process; call line to report test results and provide post-testing counselling |
| Specimen type | Anterior nares, oral fluid, aerosols | Mid-turbinate nasal swab |
| Sample collection and processing | Integrated; no separate sample extraction procedure | Swab, spit or exhaled breath sample collection and minimal sample processing using disposables (tubes, diluents) provided as part of the test kit; not more than one manual sample preparation step required |
| Number of timed steps | 0 | 1 |
| Time to result
(total turnaround time including all testing steps) |
≤20 min | ≤40 min |
| Detection | High-contrast, clear positive/negative results for naked eye, indoor and outdoor reading (visual readout) or digital readout via smartphone application | Readout using a small, portable or hand-held, battery-operated device, ≤5 kg, with data export and connectivity capabilities |
| Quantification | Quantification not required | |
| Quality control | Internal control for sample quality and process control (for sample flow/migration) in an area or region within the individual testing device | Process control (for sample flow/migration) in an area or region within the individual testing device |
| Invalid rate | ≤0.5% invalid results with correct use by operator | <2% invalid results with correct use by operator |
| Operating conditions | 10–40°C; 25–90% relative humidity up to 3000m | 10–35°C; 25–80% relative humidity up to 1500m |
| Test kit stability and storage conditions
(estimated based on accelerated stability studies) |
18–24 months at 4–40°C; tolerates
freezing and brief periods 45°C; humidity 75% + 5%
Any associated equipment must meet or exceed these requirements |
12 months at 4–30°C; tolerates brief periods >40°C; humidity 75% + 5%
Any associated equipment must meet or exceed these requirements |
| Stability of the kit once opened | 1 hour for single use test after opening the pouch | 30 minutes for single use test after opening the pouch |
| Waste management | Disposal of used testing materials in routine waste stream | |
| Connectivity | Digital connectivity through smartphone application, ability to report to country health information management systems using an onboard unique identifier or other personal data protection safeguard, linking the test to the user (e.g., QR codes, 2-D barcodes etc). | Not required |
| Manufacturing | ISO 13485:2016 compliant | |
| Analytical sensitivity
(professional use version) |
equivalent to 104 genomic copies/mL or Ct ≈>30 | equivalent to 106 genomic copies/mL or Ct ≈25–30 |
| Analytical specificity
(professional use version) |
Assay detects all circulating SARS-CoV-2 viral strains and does not cross react with common interfering substances or other human coronaviruses or any other common human diseases especially those presenting with
similar signs and symptoms of COVID-19 (e.g. influenza A, B, RSV, malaria, dengue) |
Assay detects all circulating SARS-CoV-2 viral strains and does not cross react with common interfering substances or other human coronaviruses (except SARS-CoV-1) or any other common human diseases, especially those presenting with similar signs and symptoms of COVID-19 (e.g. influenza A, B, RSV , malaria, dengue) |
| Clinical sensitivity
(professional use version) |
≥90% | ≥80% |
| Clinical specificity
(professional use version) |
>99% | ≥97% |
| Concordance of self-administered with professional use test | >99% | ≥95% |
| Test COGS* | <$1 USD | <$3 USD |
*COGS: cost of goods sold, ex-works (not including shipping and distribution margins) for individually packaged product containing all materials required for testing and digital support tools
_____________________________________________
Appendix 2: Governance, eligibility, and partner expectations
| This RFP will be executed according to FIND governance, policies and procedures. These are summarized below; full details can be found on the FIND website. Proposals and partnerships selected for funding negotiations will then engage with the corresponding donors, who will follow their own contracting and monitoring procedures. |
Low- and middle-income country access and quality
Applicants are expected to commit to:
- Abiding by the FIND global access policy.
- Supplying a minimum volume/share of self-tests produced6</sup to LMIC organizations, public or private, at affordable prices. Details to be negotiated.
- Submitting a product to WHO prequalification programme and/or stringent regulatory authority, as relevant.
- Establishing and sustaining the highest IVD quality standards, in particular during production scale-up.
- Undertaking activities in LMICs to support market introduction and access (e.g., customization of supporting materials, local registration, sales, and distribution activities).
- Committing to support the quality of self-testing, especially through proactive post-market surveillance and feedback from end-users and governments.
Product
- The proposed self-testing solution needs to target the key requirements specified in Appendix 1. Preference will be given to those that meet or have high potential to meet optimal requirements. The starting point for these product requirements is the existing WHO TPP for point-of-care tests for suspected COVID-19 cases and their close contacts, which includes self-administered tests as a “desirable” characteristic.7 If the WHO publishes a more descriptive TPP, any product development funded under this RFP should align to it.
- Products with or without accompanying digital supports tools are welcome. Willingness to partner for digital component development, or to customize an existing digital tool, is required.
- The proposal should include evidence to support product performance claims, with a large evidence base being advantageous. Products in the late stage of development are preferred.
- This RFP focuses on complete diagnostic tests. Developers of innovative components (e.g., unique sampling devices) may partner with IVD manufacturers and apply with a full IVD test offering.
- Products should be developed in accordance with ISO 13485:2016, within a QMS framework, and should be internally validated.
Organizational
- Evidence of institutional commitment and ability to act quickly, e.g., senior management, board-level, or key stakeholder support, should be provided.
- Track record of the organization or key personnel suggesting an ability to effectively implement proposed activities, including self-testing experience, should be provided.
- The proposal should demonstrate a long-term intention to sustain business beyond the COVID-19 programme.
- Applicants should be open to ongoing monitoring of the programme (e.g., access targets, business sustainability, and quality).
_____________________________________________
6 Fulfilled by reserving in-house production capacity for LMICs, or through innovative schemes that foster regional production of RDTs in LMICs.
7 In 2020, WHO published a TPP that includes among the “desirable” characteristics the option for self-administration or for administration by a trained layperson (https://www.who.int/publications/m/item/covid-19-target-product-profiles-for-priority-diagnostics-to-support-response-to-the-covid-19-pandemic-v.0.1)
Quick links