Expression of interest

Driving equitable access to fit-for-purpose antigen-detecting rapid diagnostic tests for COVID-19
This expression of interest (EOI) is now closed. The call for proposals opened on 4 July 2020, with a first deadline for receipt of submissions closing on 24 July 2020, a second on 24 August 2020. This was followed by rolling submissions being accepted until 30 October 2020. We received 115 applications from 25 countries representing 6 continents. In the event that additional funding commitments are secured at a later time, this EOI may be reopened. FIND and its partner on this call for proposals, Unitaid, are finalizing the review process; selected submissions will be announced on this site, once the agreements have been executed.
The EOI was prepared in the context of the Access to COVID-19 Tools (ACT) Accelerator Diagnostics Pillar, launched by FIND and Unitaid, and supports the work plans of two working groups within the pillar: “R&D of tests & digital tools” (led by the Bill & Melinda Gates Foundation and the Praesens Foundation) and “Market readiness” (led by Unitaid and FIND).
Background
Since the beginning of the COVID-19 pandemic, molecular testing has played a critical role in the management of suspected cases as well as contact tracing worldwide. Testing capacity, however, remains highly centralized, and often insufficient to meet the current demand. While countries in all regions have experienced these challenges, the needs are more acute in low- and middle-income countries (LMICs), where fragile health systems and exclusive reliance on global supply chains have often left LMICs unable to access much-needed tests. This situation is further compounded by the lack of reliable, affordable and easy-to-use rapid tests for clinical diagnosis of COVID-19. While such tests would be beneficial to high-income countries (HICs), they are critical for establishing testing programmes in LMICs.
An estimated 500 million COVID-19 diagnostic tests will be needed in LMICs over the next 12 months, 75% of which in decentralized settings (i.e. primary healthcare, community-level care, hospital triage). There is emerging consensus about the important role that SARS-CoV-2 antigen-detecting rapid diagnostic tests (Ag RDTs) will play in filling this gap, as the primary diagnostic for active infection detection in decentralized settings where timely molecular testing is not available.
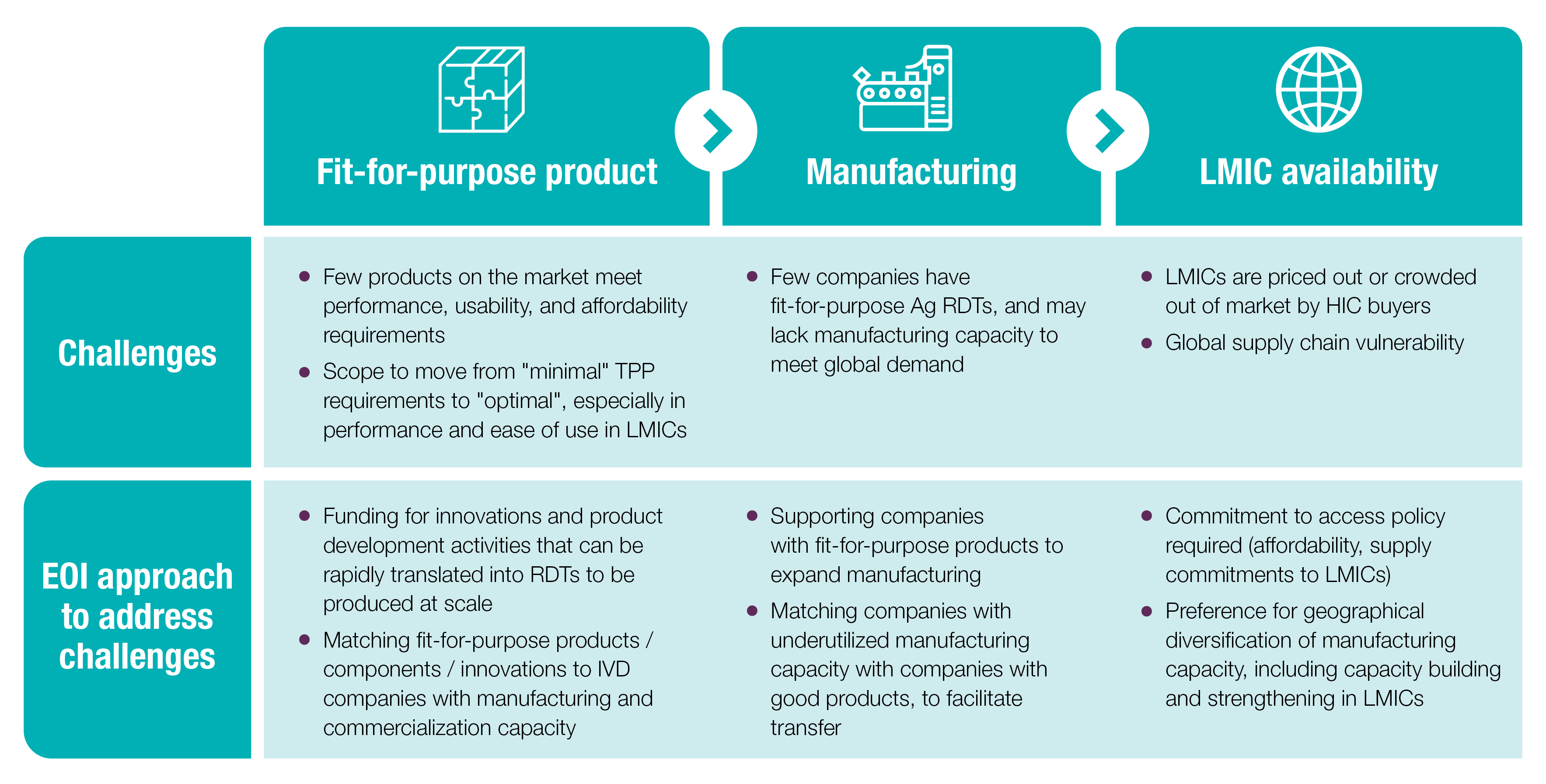
While the situation is dynamic, several market challenges stand in the way of supplying LMICs with fit-for-purpose Ag RDTs:
Scope
This project aims to fill current diagnostic gaps in the detection of active SARS-CoV-2 infection for case management and contact tracing purposes in decentralized settings. In this context, a test that detects the virus in a user-friendly, field-appropriate format is desirable. While both point-of-care nucleic acid testing platforms and Ag RDTs would be appropriate in these settings, this project is focussed on accelerating supply and availability of quality-assured and regulatory-approved fit-for-purpose Ag RDTs.
The ACT-Accelerator Diagnostics Pillar (ACT-A Dx) will engage end-to-end, from the development and deployment process to driving access to these fit-for-purpose Ag RDTs. This will include accelerating development, facilitating regulatory approvals, and increasing production capacity, addressing supply chain challenges, establishing mechanisms (such as volume guarantees) to de-risk the market, and strengthening local capacity for development and deployment of new tests within national testing strategies. This EOI is just one of several mechanisms being developed by ACT-A Dx, and is focused on the downstream activities of product development and manufacturing scale-up.
Proposals and business partnerships are sought in two areas:
- Accelerating development and market entry of improved, quality-assured SARS-CoV-2 Ag RDTs for expanded use in LMICs
- Rapidly creating the supply conditions (manufacturing capacity, diversity of supplier base, affordability) to meet the needs of LMICs
Benefits
An initial budget envelope of up to US$40 million of grant funding, to be made available by FIND and Unitaid and supplemented by loan funding from development banks, could support at least 2–4 proposals that offer best value for money. As additional funding becomes available, further proposals will be considered. Funding negotiations will be conducted independently for each proposal, and will be tailored to the applicant’s needs and the specifics of each business case. Funding could take many forms, such as R&D grant funding, loans for infrastructure scale up, licensure agreements, and/or longer-term volume commitments.
Alongside financial support, the direct benefits of this programme include partnership opportunities and advisory/technical support.
Proposals were sought from innovators, RDT developers, IVD manufacturers, and LMIC-based diagnostic stakeholders interested in:
- Expanding their own production capacity, with commitment to LMIC supply
- Supporting geographic diversification of manufacturing in one or more LMIC regions with local partnerships, including (but not limited to) contract manufacturing for open-label products
- Partnering with readily available high-quality manufacturing capacity globally, or offering their own excess production capacity to other developers
- Licensing and/or technology transfer
More information
For more information please contact us.
online workshop co-hosted by find & unitaid, 10 july 2020 @ 07h00
online workshop co-hosted by find & unitaid, 10 july 2020 @ 17h00
About the Access to COVID-19 Tools (ACT) Accelerator
The ACT Accelerator is a ground-breaking global collaboration to accelerate the development, production, and equitable access to COVID-19 tests, treatments, and vaccines. It was set up in response to a call from G20 leaders in March and launched by the WHO, EC, France and the Bill & Melinda Gates Foundation in April 2020.
The ACT-Accelerator is not a decision-making body or a new organization, but works to speed up collaborative efforts among existing organizations to end the pandemic. It is a framework for collaboration that has been designed to bring key players around the table with the goal of ending the pandemic as quickly as possible by reducing COVID-19 mortality and severe disease through the accelerated development, equitable allocation, and scaled up delivery of tests, treatments and vaccines, thereby protecting health systems and restoring societies and economies in the near term. It draws on the experience of leading global health organizations which are tackling the world’s toughest health challenges, and who, by working together, are able to unlock new and more ambitious results against COVID-19. Its members share a commitment to ensure all people have access to all the tools needed to defeat COVID-19 and to work with unprecedented levels of partnership to achieve it.
The ACT-Accelerator has four pillars of work: diagnostics, therapeutics, vaccines and the health system connector. Cross-cutting all of these is the workstream on access & allocation.
Quick links